Date
- Sep 15 2023
- Expired!
Time
- 1:00 pm - 2:00 pm
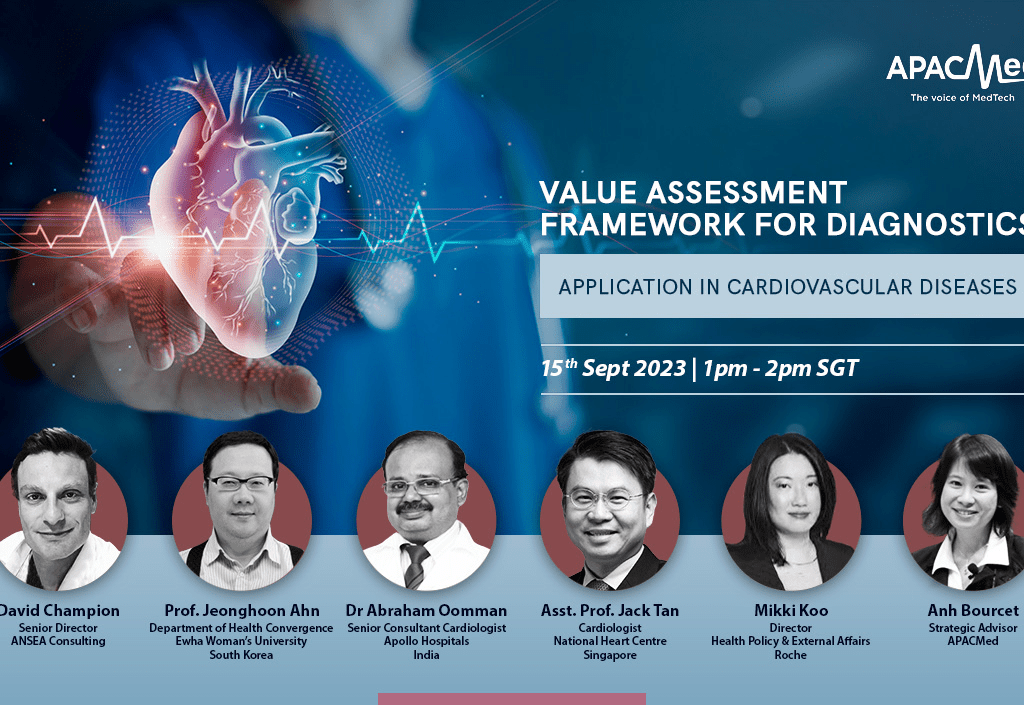
Value of Diagnostics Assessment Framework Paper Launch Webinar
Diagnostics are the gateway to care, however less than half of the world population have access to these technologies. The 7th World Health Assembly Resolution on Strengthening Diagnostics Capacity (2023) marks a major milestone in recognising the critical role they play at all levels of health care.
The APACMed IVD Working Group has initiated advocacy work since 2022 to raise awareness to decision-makers in the Asia Pacific on the “Value of Diagnostics”, for better patient access to diagnostic innovations. Building on the first 2 publications, this paper aims to continue the conversation, advocating for enhanced recognition of the substantial value in-vitro diagnostics provide to patients, healthcare professionals, providers/laboratory professionals, policy-makers and payers in the Asia Pacific region. In particular, it highlights the need for a comprehensive fit-for-purpose value assessment framework for in-vitro diagnostics (IVDs), and proposes such a framework in the case of cardiovascular diseases (CVD) to illustrate.
The purpose of the webinar, therefore, is to serve an initial launch of the paper as a means to educate the audience about recommendations to better evaluate IVDs illustrated in the case of CVD.